NEW EFFICIENT AND ECONOMIC MOVING BED PROCESS (MBBR) FOR THE REDUCTION OF NITRATES (NO3) IN GROUNDWATERS
NEW EFFICIENT AND ECONOMIC MOVING BED PROCESS (MBBR) FOR THE REDUCTION OF NITRATES (NO3) IN GROUNDWATERS
How to reduce nitrates in groundwater
Nitrates (NO3) and nitrites (NO2) are inorganic ions which exist in nature in small quantities, as part of the natural nitrogen cycle in the soil. They are released by rain from the terrain (forests, minerals and rocks) into rainwater runoff. In rivers and subterranean flows that are not affected by human activity, the normal concentration of NO3 tends to be around 10 ppm, but in flows near places with industrial activity this concentration can grow to high and excessive concentrations, which can be dangerous to the health of animals and humans if ingested.
INTRODUCTION
The main cause of high NO3 in subterranean waters tends to be liquid wastes coming from animal husbandry (porcine, ovine and bovine mainly). In places where the animal wastes have been used without control as a fertilizer, concentrations have been shown to reach up to 180 ppm of NO3 in groundwater.
A study conducted by researchers from the Univeristy of Vic (Barcelona) -published in the magazine Tecnoaqua in October 2013- states that twelve counties in Catalonia suffer from high NO3 contamination in their subterranean waters and fountains. These counties all share a high level of cattle activity, except for one where there are many plant cultivation areas. Furthermore, it is known that Belgium has more than 65% of its groundwaters contaminated with nitrates, as happens in other European countries and states in the American Middle West.

Since the tests that are needed to determine the amount of nitrogen that can be absorbed by a certain soil type are not always performed, overuse often results. Together with the rain, this causes the contamination of lower soil strata and groundwaters. Soil NO3 leaches very easily, moving quickly to the lower strata of the soil.
CONSEQUENCES OF HIGH CONCENTRATIONS OF NO3 IN THE WATER
High concentrations of NO3 in food are extremely harming to the health of the animals, including humans. The World Health Organization has established a maximum tolerable limit of 50 ppm. NO3 complicates the oxygenation of the blood becoming nitrites (NO2). Hemoglobin mutates to metahemoglobin and, as a consequence, less oxygen reaches the organs and tissues. This can cause the death of fetuses in pregnant mammals and, in human babies, it can produce the so-called “blue baby” effect (lack of oxygen in the tissues).

HOW DOES THE NEW BIOLOGICAL ANAEROBIC TREATMENT FLUIDIZED BED SYSTEM USING ADAPTED MICROORGANISMS FUNCTION?
This system reproduces the natural denitrifying cycle. A very successful pilot experience has been performed with well water from a cattle farm, which had a NO3 concentration of over 160 ppm, and achieving after the treatment an effluent with 4 ppm of NO3. The result of this experience has culminated in a first industrial plant to treat well water from a ranch with over 200 head of cattle.
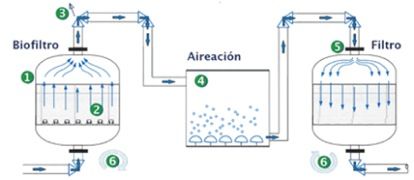
The system consists in a first anaerobic continuous flow reactor (1) that contains a fluidized plastic media bed with a high specific surface area (MBBR) (2) where naturally-occurring bacterial colonies grow along with specialized bacteria (BACTERIA®- DEN Microbial Cultures) added to the system. Here the transformation of NO3 to N2 gas occurs under anoxic conditions with the help of an energy source (organic matter) and nutrients. Nitrogen gas is discharged into the atmosphere (3).
In a second aerobic activated sludge reactor (4) the excess organic matter is biodegraded (the organics contained in the influent, added in the denitrification process, or having been generated by the biotransformation of compounds inside the anoxic system).
Finally, a filter system (5) separates solids and water producing an effluent which is clarified and almost nitrate free (6).
Denitrification occurs by the action of facultative heterotrophic bacteria of the types: pseudomonas (group 1, non pathogenic), paracoccus and alcaligenes. These bacteria are naturally occurring in the environment and are harmless for humans and animals.
The transformation process of nitrates to nitrogen gas happens in a series of steps catalyzed by different enzymatic systems. During the process, different intermediate products like nitrites (NO2), nitric oxide and nitrous oxide (NO) are produced:
This process, that would take a long period of time to recover if the input of nitrates is stopped suddenly, is made in a fast and efficient way by maintaining within the process large quantities of biomass on a carrier (plastic media), where the colonies of bacteria are sheltered and reproduce (BIOFILL® TYPE-C).
The plastic media BIOFILL TYPE C is one of the best carriers available due to its high specific surface area (460 m2/m3), wrinkled surface which allows high biomass adhesion and large free volume (>90%). This allows good circulation of the fluids in the system and easy detachment of dead biomass.

The efficiency of this process is enhanced by the input of preselected, cultured denitrifying microorganisms that perform under anoxic conditions. These are a combination of anaerobic facultative microorganisms selected from the environment for their capacity to use nitrates as nutrients.
Under proper conditions, NO3 and NO2 ions accumulated in the water are broken down by the cultures in BACTERIA®- DEN. These cultures augment the colonies of indigenous denitrifying bacteria in the initial phase of the treatment, accelerating the elimination of NO3 and NO2 ions and contributing to the maintenance of an optimal colony of microorganisms in the system.
Also, if the system stops for any reason, the addition of BACTERIA®- DEN reactivates the biological system quickly in 48-72 hours. For system start up about 9-10 g. of BACTERIA®- DEN/m3 of water are used, and about 3 g/m3 for preventive maintenance.
The ideal conditions needed to induce denitrification are as follows:
- High presence of denitrifying bacteria in the process.
- Available nitrogen in oxidized forms (NO3; NO2).
- Absence of dissolved molecular oxygen.
- Presence of oxidable organic matter (DBO5), like methanol, acetic acid or others.
BACTERIA®- DEN is also specially helpful for difficult industrial waters containing amines, municipal waters, food and chemical industry waters, and fish farms.

ADVANTAGES OF THE SYSTEM
The systems are modular and made with simple, quality components. Installation and operating costs are low (lower than other systems in common use now), and the system does not generate wastes or odors.
COMPARATIVE TABLE ON OTHER PROCEDURES FOR NO3 ELIMINATION
| PROCEDURE | REVERSE OSMOSIS | IONIC EXCHANGE | BIOFILTRATION |
|
Operating cost |
HIGH; increasing as the working hours increase |
MEDIUM |
LOW; around 4-5 times less than reverse osmosis |
|
Removal |
50-60 % |
100 % |
95% |
|
Efficiency |
90 % |
85 % |
85 % |
|
Discharges |
Can reach up to 50% of the water treated with nitrates and the other separated elements |
Brine with nitrates |
No nitrates |
|
Wastes |
The discharge will have to be treated by a waste adviser, depending on NO3 concentration |
The discharge will have to be treated by a waste adviser, depending on NO3 concentration |
The cleaning discharge can be disposed of in the sewage system due to low organic charge, or used as a fertilizer |
|
Necessary spare |
Change of filters every 2-3 weeks |
None |
None |
|
Recharge of filterable material |
No |
Yes |
No |
|
Consumable |
Descaling Agent |
Salt |
Carbon source |
|
Energy |
High |
Low |
Low |
|
Selectivity |
None. Clears any type of salts and ions |
None. Can clear non desired ions |
Yes. Only clears NO3 |
|
Drinking water |
Leaves water without any chemical. Salts have to be reintroduced |
Risk of excess of salt in the water |
Leaves the water with its natural composition, but without nitrates |
|
Investment |
High |
Low |
Low – Medium, depending on flow |
|
Difficulty of Operation |
Complex |
Medium |
Low |
PARTNERS IN THE EXPERIENCE
NONO3 – System design and construction of the pilot and full-scale.
Biología y Filtración S.L. – Fabrication and supply of the plastic media BIOFILL Type C- (biomass carrier) for the moving bed.
Biología y Filtración S.L. – Supply BACTERIA®- DEN Denitrifying Cultures produced by Bioscience, Inc (Allentown, Pennsylvania- USA).